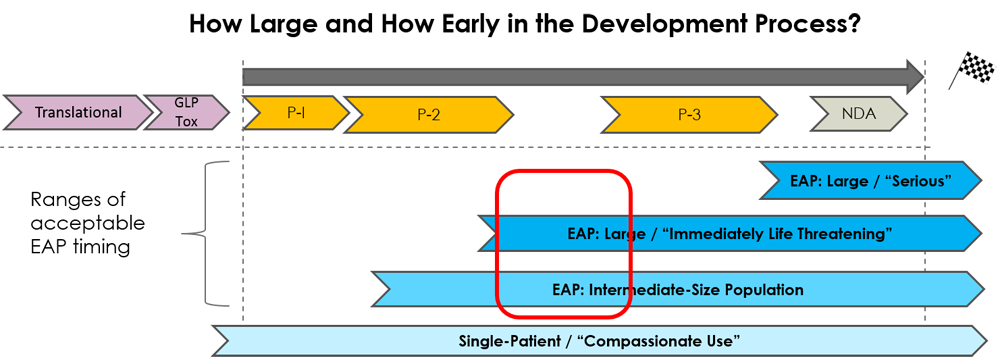
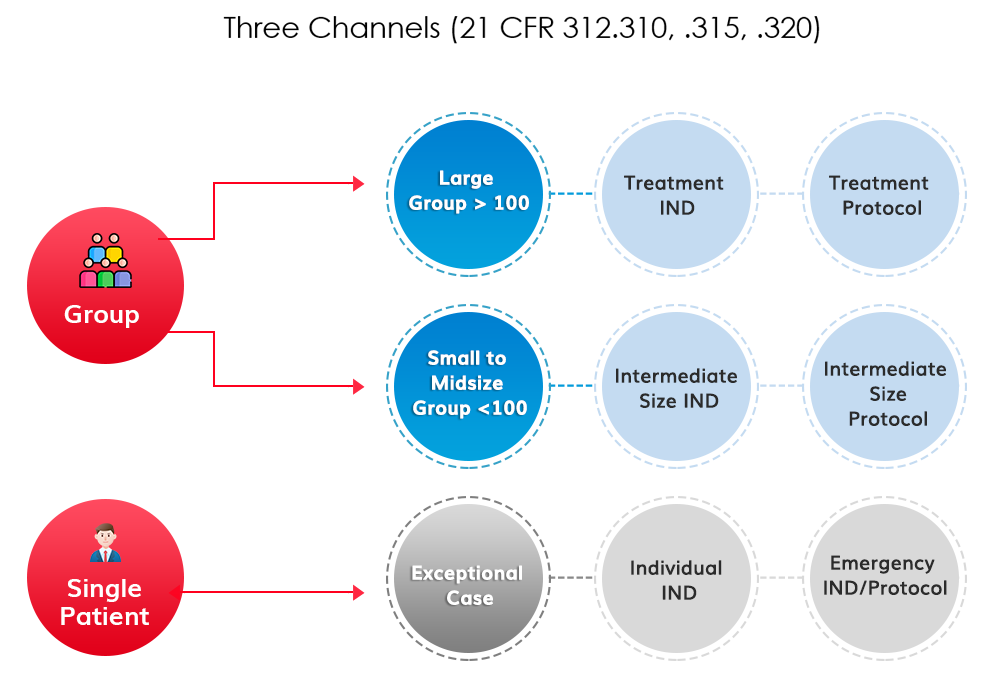
Group-level Expanded Access clinical trials (not to be confused with single-patient “compassionate use”) allow meaningful numbers of patients and their physicians to explore a new investigational treatment with a well designed protocol and supply-chain to work in harmony with the continued clinical development of the particular treatment.
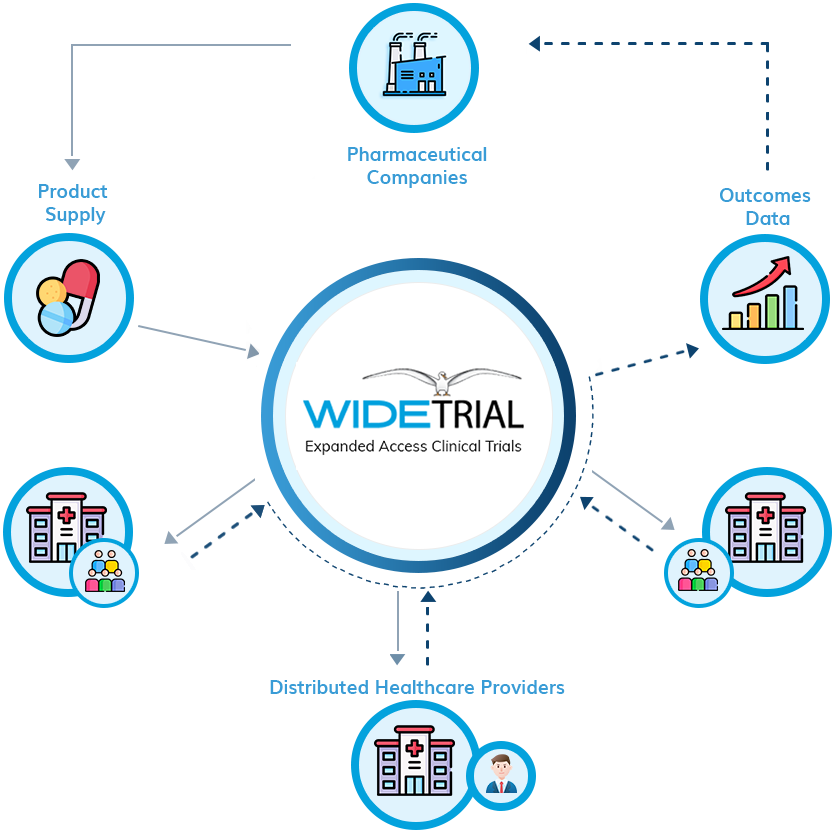
When integrated into the drug development cycle, Expanded Access trials bring many benefits to the drug company, including wider patient engagement, increased chances of discovering response-predictive biomarkers, and information that leads to more-highly targeted pivotal trials in historically difficult diseases.
WideTrial sponsors both large group and small to medium sized group EAPs. The allowable size and timing of the program depends on many factors, including the degree of medical need, the safety profile of the product, and the quality of existing clinical data. We have seen every kind of case in the 30 year history of U.S. Expanded Access. We’ll devise the right access strategy for your product.


 Past meetings: 2017, 2019, and 2020
Past meetings: 2017, 2019, and 2020 National Press Club, Washington D.C.
National Press Club, Washington D.C.



 info@widetrial.com
info@widetrial.com 2445 Augustine Drive, Suite 150
2445 Augustine Drive, Suite 150