An Expanded Access Program (EAP) for MN-166 in ALS
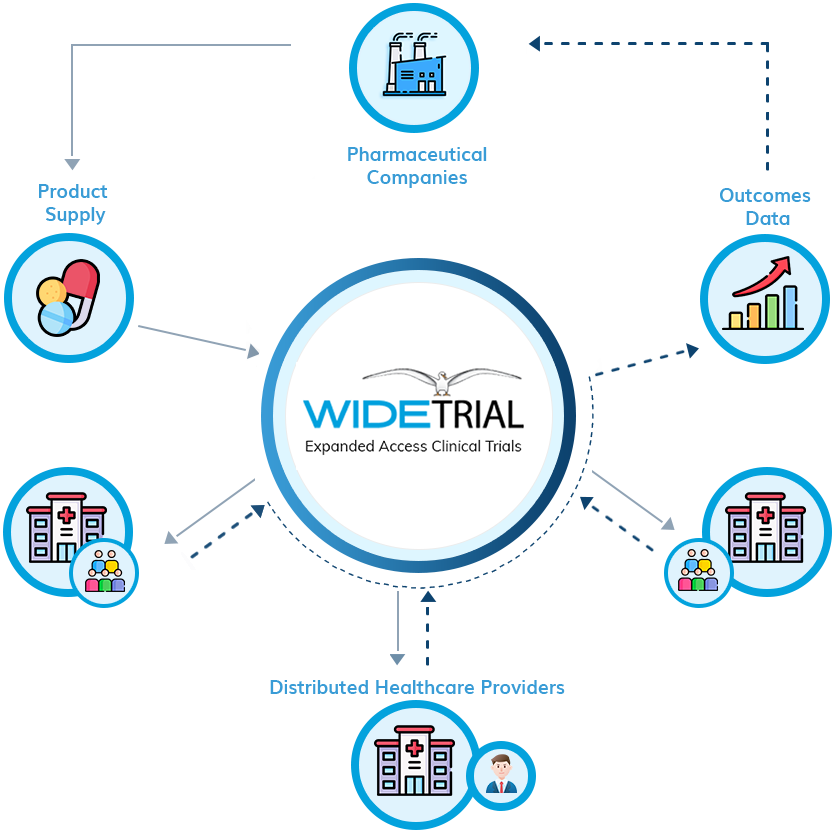
WideTrial is pleased to support the NIH-funded Expanded Access Program to provide treatment-use of MN-166 (ibudilast) for people with ALS who cannot enroll in the drug’s ongoing research trial. In partnership with a leading academic medical center, WideTrial is establishing an open network of healthcare providers who seek to bring this investigational treatment option to their patients. If you would like to learn more about participating in this EAP, please register your interest here.
Announcement: July 10, 2025Announcement: November 19, 2024


 Past meetings:
2017, 2019, and 2020
Past meetings:
2017, 2019, and 2020 National Press
Club, Washington D.C.
National Press
Club, Washington D.C.







 info@widetrial.com
info@widetrial.com 2445 Augustine
Drive, Suite 150
2445 Augustine
Drive, Suite 150